Pharmaceutical services
Patient support program design & delivery
Calian is a leading provider of patient support programs. Supporting patients through their treatment journeys with personalized and high-quality care

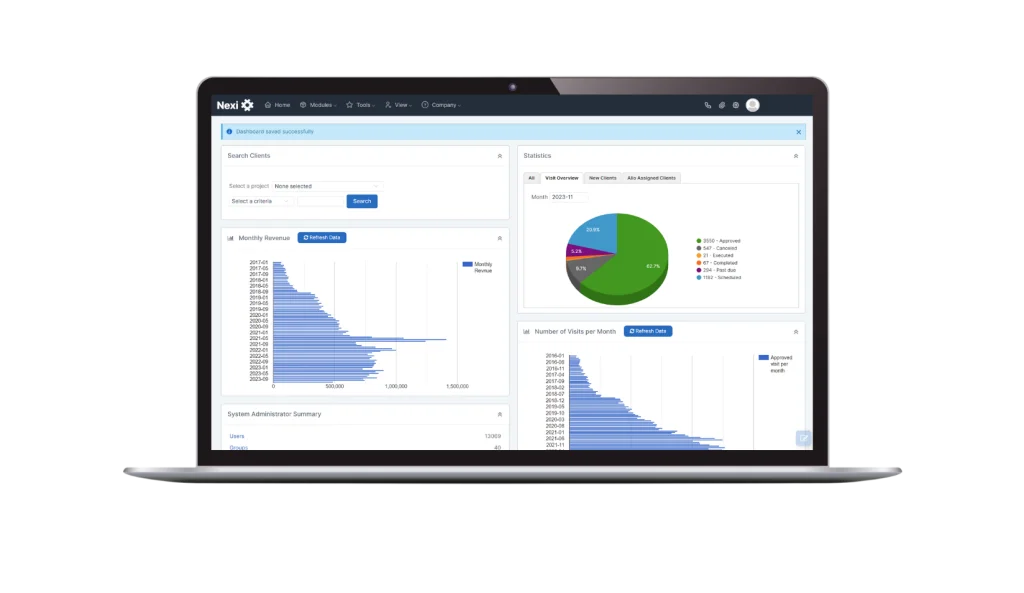
Calian® Nexi™
Nexi, our proprietary PSP workflow-automation software, plays a pivotal role in supporting patient support programs. With Nexi, we ensure stringent quality control, achieve notable cost efficiencies, and facilitate a greater volume of high-quality patient interactions.
Why choose Calian for your patient support program?

Pharmacy-inclusive models
Our patient support program offers pharmacy-inclusive models, allowing patients to choose their preferred pharmacy for convenience and flexibility in accessing medications. This patient-centric approach improves the overall experience and boosts treatment plan adherence.

Technology-enabled PSPs
We use technology to streamline patient support program administration, freeing up healthcare professionals for more meaningful patient engagement. This boosts program efficiency and improves patient satisfaction and outcomes.

Data utilization and patient registries
Our distinctive approach involves data and patient registries to gather real-world insights into patient behaviours, treatment outcomes and program effectiveness, driving continuous improvement and personalized support for superior outcomes.

Patient-centric models
Our patient support program prioritizes a patient-centric approach, tailoring support services to individual needs and preferences. This focus enhances engagement, treatment plan adherence and overall health outcomes.
Collaboration with CRO business
What sets us apart is our integration with our contract research organization (CRO) business throughout the product lifecycle. This alignment maximizes the impact of patient support programs, contributing to product success and positive patient outcomes.
The management team
Protect your sensitive data with robust cybersecurity
“Those in cybersecurity know that incidences nearly always occur over the weekend or in the middle of the night, so it has given us a level of comfort in knowing we have 24-hour monitoring of our systems.




Explore our innovative healthcare solutions
For more than 20 years, we’ve been providing sophisticated, cost-effective solutions that help people lead healthy lives.
Social responsibility at Calian
Committed to social responsibility.
ESG—more than a buzzword.
Our vision builds on our mission, values, historical commitment to social responsibility and key competencies. It provides a framework and focus for our activities and corporate communications related to ESG (Environmental Social Governance).
Download ESG report
Your service inspires us.
Proudly welcoming 100+ veterans to our team every year
Their expertise enriches our solutions, and hiring military spouses is our heartfelt way of giving back to the defence community. We’re committed to being there for you, in and out of uniform.
Explore opportunities

Relocating? We’ve got you covered.
4,000+ family members connected with family doctors
Our partnership with the Canadian Forces Morale and Welfare Services ensures that military family members are connected with family doctors, hassle-free.
Learn more

Building a resilient community together.
$650,000+ in corporate giving
We are woven into our community’s fabric, our support extending to renowned organizations and causes. We’re not just about business; we are about building a supportive and resilient community together.
Learn more

Engaging in research that matters.
Our contributions to significant research projects and our focus on environmental safety are unwavering. We’re front-runners in compliance with environmental laws and continually innovate to lessen our ecological footprint.
Learn more

Committed to exceeding expectations.
30+ Indigenous community engagements
With a published Indigenous engagement policy, Calian sets out the framework and guidelines for all Calian business activities while being wholly committed to meeting—even exceeding—the five per cent Indigenous participation requirement on all Federal contracts.
Learn more
Global innovation for connected, safe and healthy lives
Explore our four business units below.